|
Mouse Cardiovascular Development Atlas Construction Using EFIC Imaging
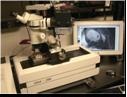
Introduction to EFIC Imaging
Episcopic fluorescence image capture (EFIC) is a novel imaging technique used to generate serial section images of biological specimens. Unlike other imaging techniques, such as MRI and CT scan, EFIC generates high resolution images utilizing only standard light microscope optics, but yet provides serial section image stacks that are in perfect registration ideal for rapid 3D reconstruction. Such perfectly aligned image stacks also can be easily resectioned in any orientation, allowing viewing of the same specimen in different virtual planes regardless of the original plane of sectioning. Moreover, with EFIC sectioning as with traditional microtomy, the tissue sections can be retrieved for further analysis with histochemical staining. EFIC imaging takes advantage of tissue autofluorescence, and entails capturing epifluorescent images of the block face of paraffin embedded tissue specimen. To prevent fluorescence bleed through from the underlying tissue layers, an aniline dye is added to the embedding medium to serve as an opaque mask. Spectral analysis shows that the fluorescence signal in EFIC imaging comes largely from NADH. Different fixatives aid in preserving the NADH emission, with 10% phosphate buffered formalin being the optimal fixative for this technique. In addition to fixation, filters play a large role in image quality. Filters with an excitation/emission of ~425/480nm provide good contrast with a low exposure time. Although paraffin is the standard embedding medium, EFIC image stacks also can be obtained with polyethylene glycol (PEG) and cryo (CRYO) embedded samples. The image quality is reduced compared to paraffin embedding, but nevertheless is acceptable, still allowing for good 3D reconstructions. Particularly advantageous is the lower temperature used for PEG (40oC) and cryoembedding (-80oC), which allows for the preservation of protein (PEG and cryo) and RNA (cryo). RNA extracted from EFIC sections collected by cryomicrotomy has been successfully used for microarray analysis.
Instrumentation for EFIC System
|